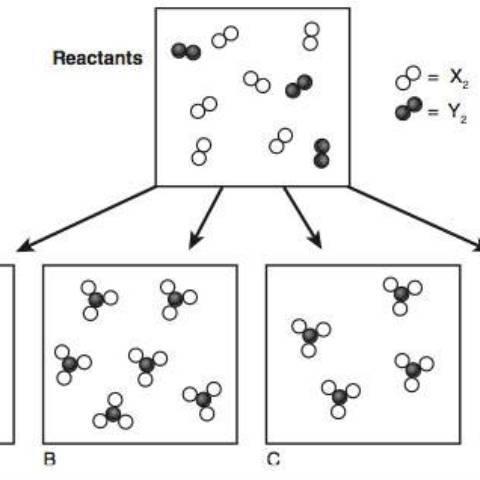
If you took an AP Chemistry exam prior to May 2014, you did not see questions like the ones shown here. In 2012, The College Board rolled out its new redesigned AP Chemistry course: a 169-page tome detailing the 6 Big Ideas of Chemistry, the 6 Enduring Understandings, the 25 statements of Essential Knowledge, the 7 Science Practices, and 117 Learning Objectives.
Before that day ended, the AP Chemistry listserv was swamped with teachers asking two questions: “Does anyone know what a PES is?” and “Where can we access these particulate views of chemical reactions?” In shifting the focus of the course away from the traditional, more algorithmic approach, The College Board had inserted concepts that were so new that they had not made it into the latest college textbooks. Although they promised that video lessons and practice questions on the new topics would soon be available, these materials were not available until midway through the 2013-2014 school year when the redesigned course went into effect.
As teachers found resources, they posted them on the listserv. Professors at two universities (University of Arizona and Oklahoma State University) made parts of their websites available for free. By Christmas vacation, Pearson Publishing had added the new concepts and posted activities to their Mastering Chemistry website for those who use their e-texts. (An advantage of e-texts is that they can be updated easily without having to publish a new printed edition of the textbook.) It turned out that PES (Photoelectron Spectroscopy) was relatively easy for students to grasp; however, the particulate representations (surprisingly) were more difficult to master.
In June, I had the opportunity to attend the AP Chemistry Institute at Texas Christian University in Fort Worth. This institute was led by veteran chief reader/table leader Dr. John Gelder of OSU. He taught us his approach to particulate discussions and gave us access to his online virtual activities designed by professors and graduate students at OSU. (Pearson began incorporating these same activities into Mastering Chemistry this summer.) We also learned how to adapt more traditional chemistry laboratory activities into the inquiry approach and how students can include particulate discussions in those lab write-ups.
Dr. Gelder also discussed the results of the AP Chemistry Exam Reading under the new framework. There were several interesting observations. The new test is too long: only 10% of the students taking the exam even got to the last question. The average scores on the free response questions were extremely low; typically, averages run about 50% of the maximum point value. This year, most questions averaged 33% or less. The last question averaged only 10%. Globally, the overall average score dropped almost 20%. Darlington fared much better: overall our scores dropped about 5% -- probably due to the length of the test. (That last question was relatively easy; more students would have gotten points there if they had gotten to the question.)
Here at Darlington, our students get frequent hands-on lab experiences. As students struggle with challenging labs, they must decide what to do next to find the solution to the problem. If they make a claim, they must support it with evidence. Learning happens as students grapple with their interpretations. We have a diverse student population that brings to the classroom new and different approaches to problem-solving. This enables Darlington students to better develop their critical-thinking skills and offers them more creative ways to solve problems.
Darlington students have a spirit of engagement in almost everything they do. That spirit helps carry them through the rough spots where others may falter. Each May, I encourage my students to “show them what you know” and “don’t give up” when taking the AP Chemistry Exam. I am always proud of them for staying with one of the most cognitively challenging courses that they can take in high school. Although I personally love the “chemistry” and enjoy sharing how to think about the “little particles,” students can gain greater insights as they learn to focus their energies to learn abstract concepts and, hopefully, to develop their own curiosity about the world around them.
I appreciate the generosity of our Board of Trustees in awarding me a grant from the Thatcher Faculty Development and Research Fund, which allowed me to travel to the beautiful TCU campus for the AP Chemistry Institute. Providing the means for teachers to hone their professional skills will ensure that Darlington will continue to develop outstanding young leaders well into the 21st Century.
Photo credits:
Figure 1
Figure 2
Figure 3